An Atom of Helium-4 Differs From an Atom of Lithium-7
Helium and lithium are two elements that differ only in one electron. Calculate the value for the mass defect of a mole of the following.
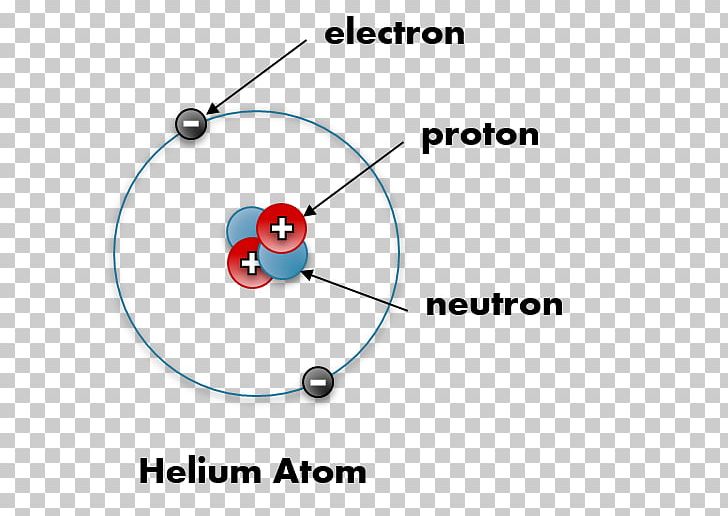
Diagram Helium Atom Proton Electric Charge Png Clipart Angle Area Atom Atomic Theory Bohr Model Free
How can the exact same collection of particles weigh less when theyre all assembled into one atom instead of two.

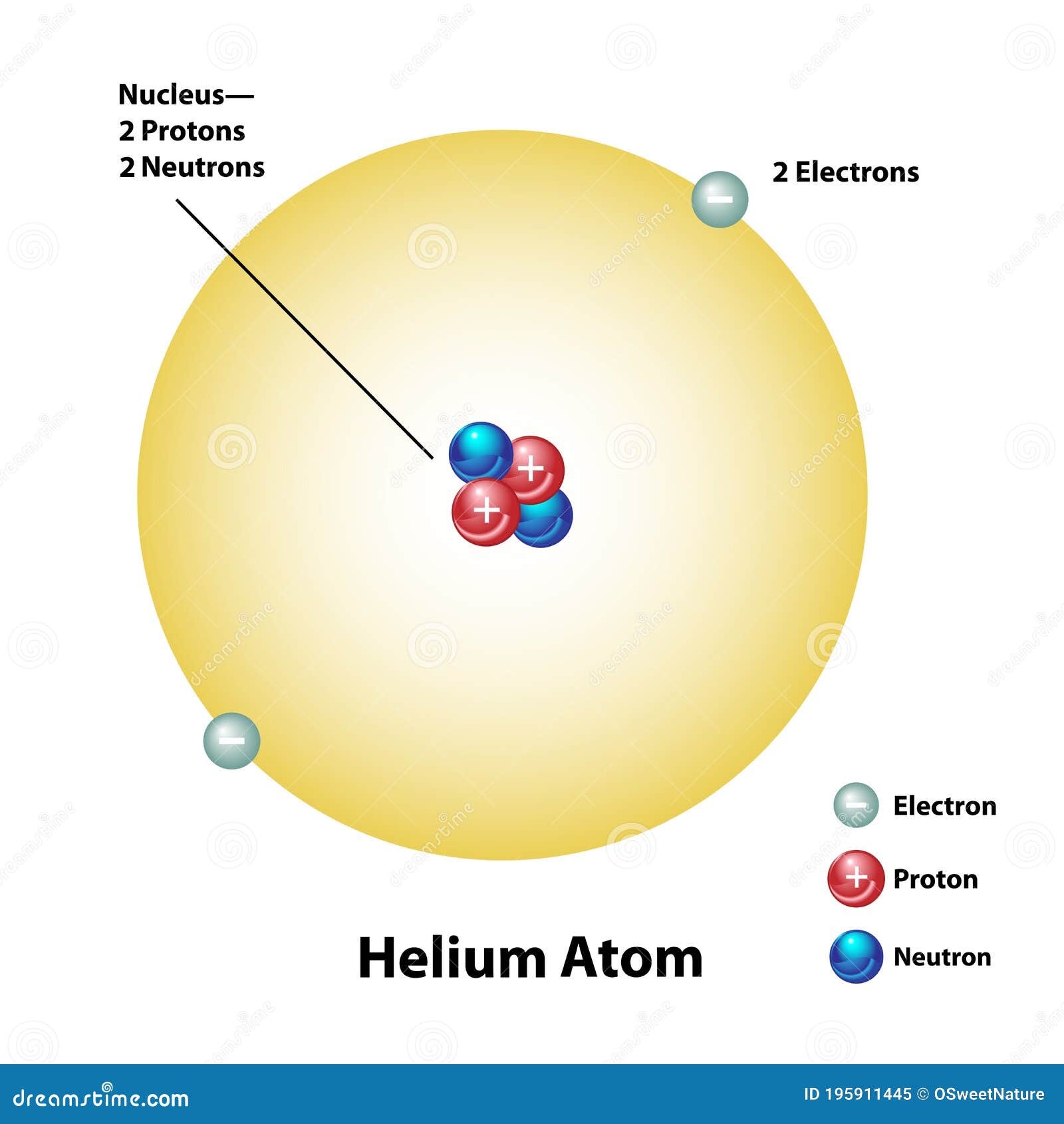
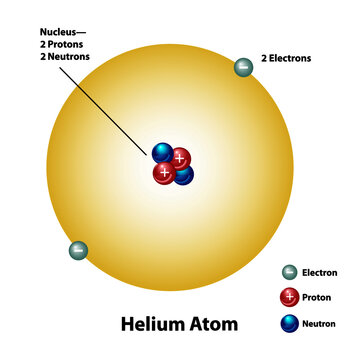
. Therefore in a helium atom there are 2 protons 2 neutrons and 2 electrons. Explain what makes one atom different from another atom. An oxygen-16 atom is about four times the mass of a helium-4 atom.
You do this by finding the mass number from the periodic table and subtracting the atomic number the number of protons you found earlier Thus hydrogen has zero neutrons helium has 2 the mass 4 minus the no. Hence we can conclude that lithium has more protons neutrons and electrons as compared to helium. What is the mass number of an atom that consists of.
All the elements of similar categories show a lot of similarities and differences in their chemical atomic physical properties and uses. Carbon 14 isotopic mass Question. Detection of LiHe was done via fluorescence.
These similarities and dissimilarities should be known while we. But keep in mind that the mass number is NOT an actual mass. Elements- Defined by Their Number of Protons.
Primordial nucleosynthesis is believed by most cosmologists to have taken place in the interval from roughly 10 seconds to 20 minutes after the Big Bang and is calculated to be responsible for the formation of most of the universes helium as the isotope helium-4 4 He along with small amounts of the hydrogen isotope deuterium the helium isotope helium-3 3 He and a very. Previously 7 Li 4 He was predicted to have a binding energy of 00039 cm 1 7710 8 eV 1210 26 J or 6 mK and a bond length of 28 Å. Beryllium and Lithium have many differences.
Naturally occurring lithium 3 Li is composed of two stable isotopes lithium-6 and lithium-7 with the latter being far more abundant on EarthBoth of the natural isotopes have an unexpectedly low nuclear binding energy per nucleon 5 33233123 MeV for lithium-6 and 5 60644016 MeV for lithium-7 when compared with the adjacent lighter and heavier elements helium 7. Therefore in a lithium atom there are 3 protons 4 neutrons and 3 electrons. Of protons 2 lithium has 4 7 - 3 etc.
If scientists count four protons in an atom they know its a beryllium atom. The more tricky bit is figuring out the number of neutrons. Atomic number of lithium is 3 Atomic mass is 7 Its a 1st group and 2nd period element Lithium is a alkali metal.
Lithium Li 7 3 4 Beryllium Be 9 4 5 Copper Cu 64 29 Boron B 11. If you get the number 146 youve sussed it. Boron 15 isotopic mass 15031088 u b.
View Notes - Discussion Forum 4 from PHY 116 at Thomas Edison State College. The total number of protons and neutrons in its nucleus is. A deuterium atom has a mass of 2014102 amu which means that 2 deuterium atoms weigh 4028204 which is 000202 amu more than the helium.
Symbolic Representations of Atoms The information you need for the following questions can be obtained from the above figures and Table 21. Other van der Waals-bound helium molecules were previously known including Ag 3 He and He 2. Now if you remove neutrons from the nuclei of any element except hydrogen they form isotopes that have similar chemical properties and different physical properties while still being an atom of the same element - therefore the protons if I understand it correctly are what determine whether an element is a gas or a solid at room.
Compare Lithium and Helium on the basis of their properties attributes and periodic table facts. The two stable isotopes of lithium are lithium-6 three protons and three neutrons in their nuclei and lithium-7 three protons and four neutrons in their nuclei. The two figures at the top are just slightly different ways to represent a lithium-7 atom.
An atom of helium-4 differs from an atom of lithium-7 in that the atom of helium-4 has. Beryllium 9 isotopic mass 9012182 u 2. A carbon-12 atom is about twelve times the mass of a hydrogen-1 atom.
Lithium 7 isotopic mass 7016004 u c. Lithium has a fairly low melting point and Beryllium has a high melting point. A helium-4 atom has a mass of 4002602 amu.
Lithium reacts readily with water where Beryllium does not. Up to 24 cash back approximate relative masses of different isotopes. Of protons 2 lithium has 4 7 - 3 etc.
Helium 4. You do this by finding the mass number from the periodic table and subtracting the atomic number the number of protons you found earlier Thus hydrogen has zero neutrons helium has 2 the mass 4 minus the no. An atom of fluorine has a mass of 19 atomic mass units.
A helium-3 atom has two protons and one neutron in its nucleus while helium-4 has two protons and two neutrons in its nucleus. Compare elements on more than 90 properties. Calculate the value for the mass defect of an atom of the following.
An atom with two protons is always a helium atom. Answer 1 of 4. Atomic number of helium is 2 and its mass number is 4.
The Nuclear Model of the Atom Lithium-7 atom 7 3 Li Lithium-7 atom 7 3 Li Part 1. The stable isotopes of helium are helium-3 and helium-4. The lithium atom in the X 2 Σ state was excited to A 2 Π.
Up to 24 cash back Hydrogen Atom Helium Atom Key Symbol Subatomic Particle Proton p Neutron n0 Electron e- Nucleus Electron Cloud Nucleus Electron Cloud. The list goes on. An atom with three protons is a lithium atom an atom with five protons is a boron atom an atom with six protons is a carbon atom.
Atom Helium Stock Illustrations 1 393 Atom Helium Stock Illustrations Vectors Clipart Dreamstime

The Atom 1 Parts Of The Atom 2 How To Draw An Atom Ppt Download
Helium Atom Images Browse 2 062 Stock Photos Vectors And Video Adobe Stock
No comments for "An Atom of Helium-4 Differs From an Atom of Lithium-7"
Post a Comment